- zohrehmasoumi17
- May 14, 2024
- 1 min read
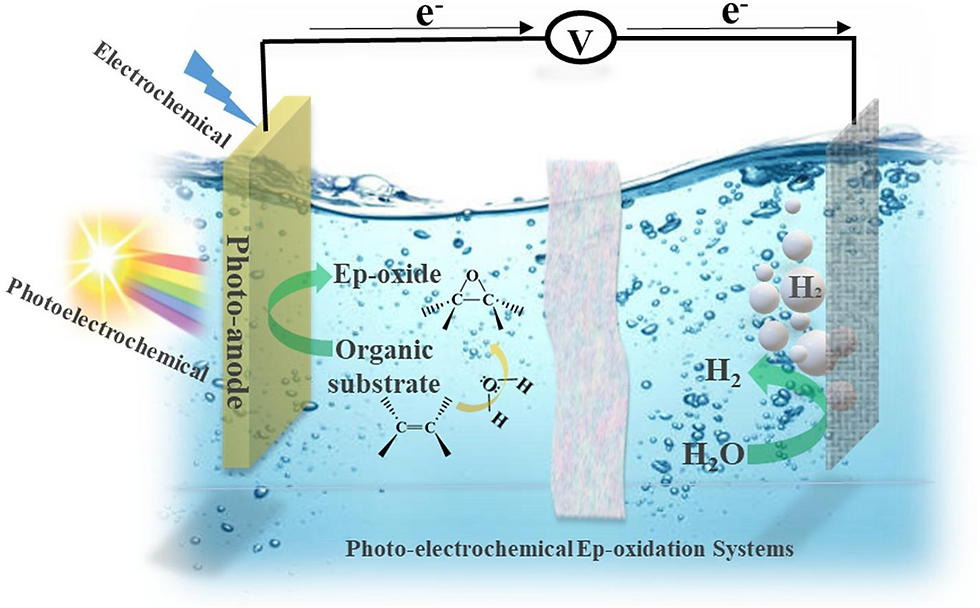
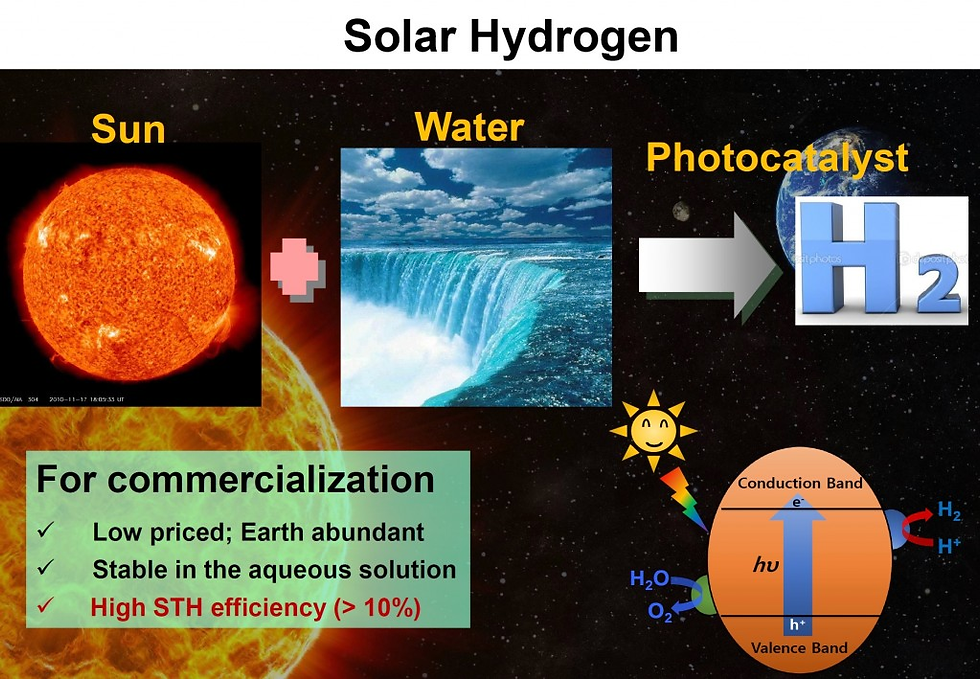
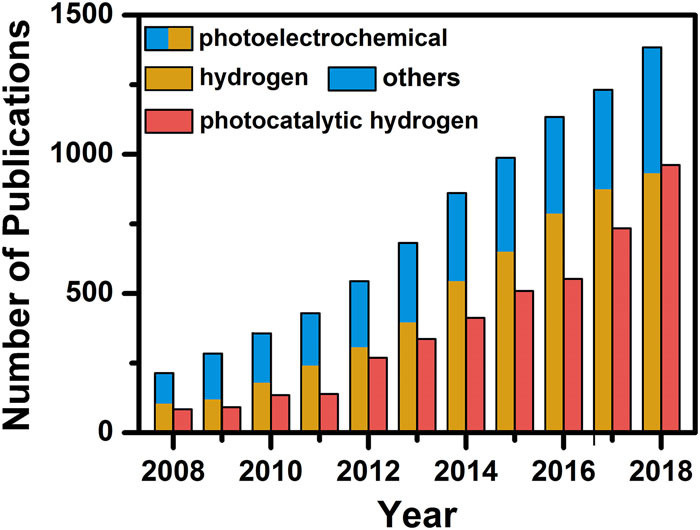
Photoelectrochemical and electrochemical (photo-electrochemical) systems for epoxidation and oxidation (ep-oxidation) are a novel, stable, and efficient methods that produce value-added products. Recently, several studies have been reported on photo-electrochemical epoxidation using environmentally friendly oxidants.
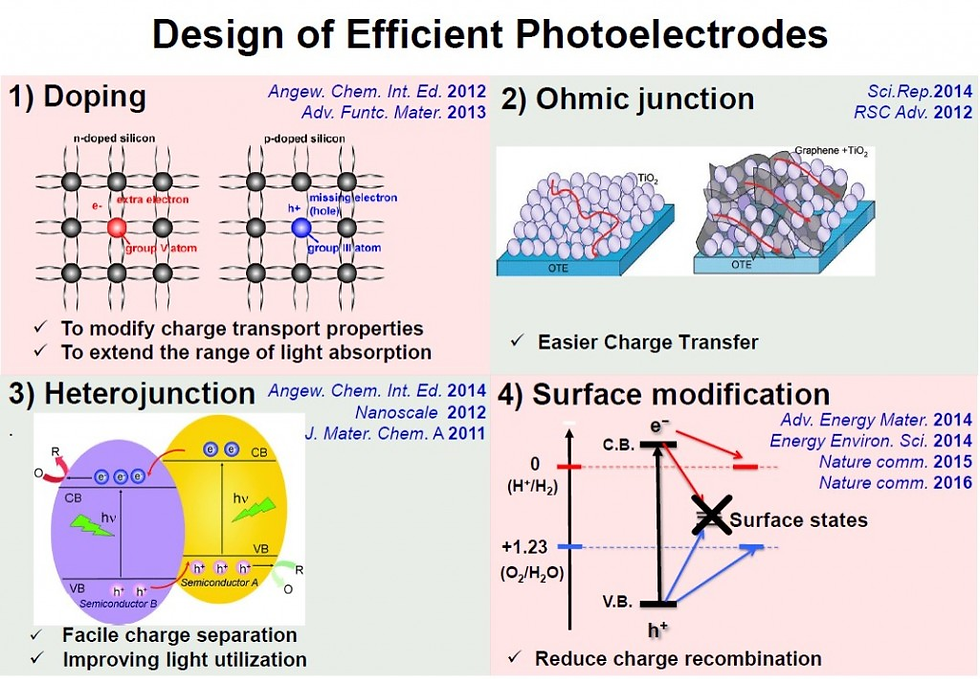
Here -> https://doi.org/10.1016/j.ccr.2023.215641, we present an overview of photo-electrochemical for ep-oxidation systems that imparts oxidizing behavior in organic molecules and relies on green oxidants, i.e., H2O and O2 as the ultimate oxygen source, which is in line with the main trend of sustainability. This review also focuses on the photo-electrochemical properties of the engineered photo-catalyst thin films, in particular synthetic outlook, efficiency, activity, and selectivity.