Design Photoelectrode for Solar Hydrogen Production
- zohrehmasoumi17
- Apr 27, 2022
- 3 min read
Updated: May 3, 2022
Solar Hydrogen Production
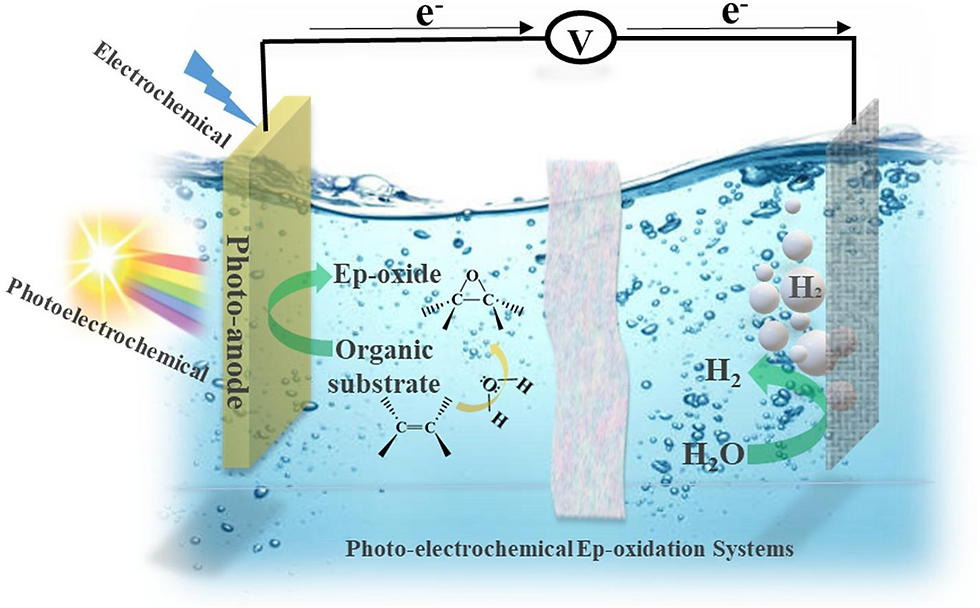
Photoelectrochemical (PEC) water splitting represents one of the most promising energy conversion processes capable of directly producing hydrogen from renewable energy sources. A typical PEC cell consists of a photoactive semiconductor as a working electrode, a counter electrode (usually Pt), and an appropriate supporting electrolyte. When an n-type semiconductor photoelectrode is used, photo generated holes migrate to the photoelectrode/electrolyte interface and perform water oxidation, while photoexcited electrons travel via the back contact and external circuit to the counter electrode, where they reduce water. In such a practice, the n-type semiconductor serves as the photoanode and its valence band level must be more positive than the H2O/O2 potential (+1.23 V vs RHE) to allow efficient oxygen evolution. The Fermi level of the photoanode, on the other hand, determines the potential of the photoexcited electrons at the counter electrode, which can be otherwise less negative than the H+/H2 potential (0 V vs RHE) since the potential deficiency can be compensated for by applying an external voltage. Similarly, a p-type semiconductor can function as the photocathode for practical hydrogen evolution, given that the conduction band level is more negative than the H+/H2 potential. Alternatively, a photoanode and a photocathode can be connected in a two-electrode cell without employing a counter electrode.
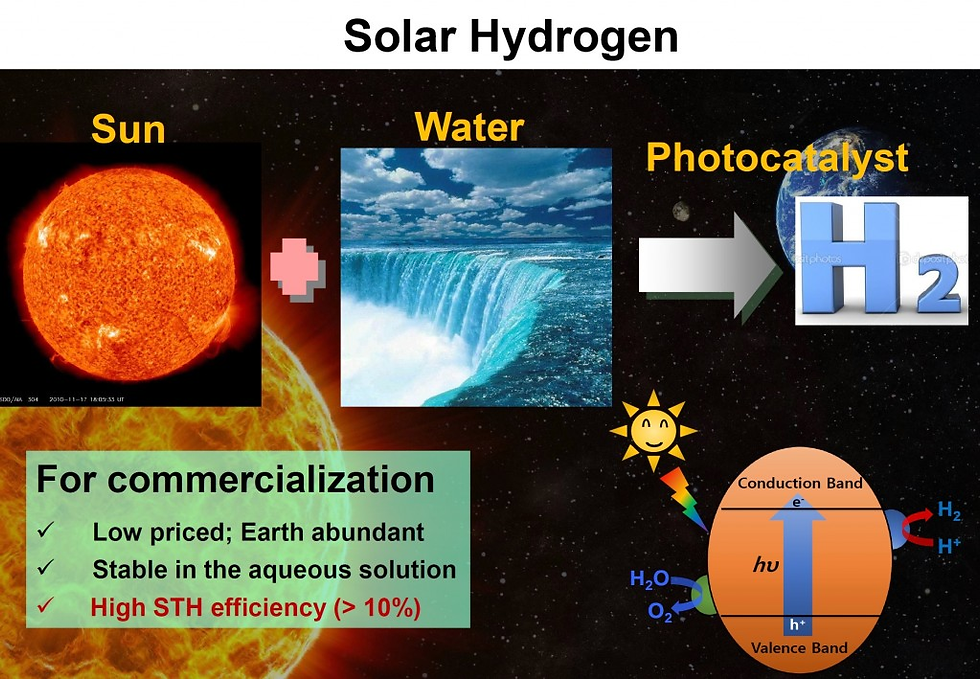
“The ubiquitous availability of water and its low-carbon footprint make PEC water splitting a green and sustainable approach to solve the problem of ever-rising global energy demand.”
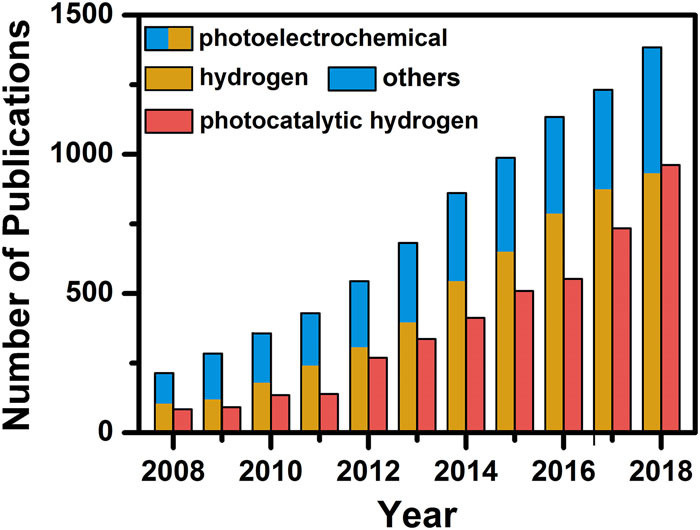
The ubiquitous availability of water and its low-carbon footprint make PEC water splitting a green and sustainable approach to solve the problem of ever-rising global energy demand. Since Fujishima and Honda demonstrated the first PEC water splitting apparatus using TiO2, the number of related publications each year has increased significantly. The high importance and tremendous interest in this field can be highlighted by a simple search on the ISI Web of Knowledge database using “PEC” and “photocatalytic hydrogen” as title keywords. As summarized in Figure, over the past ten years, the number of publications on “PEC hydrogen” and “photocatalytic hydrogen” has both exhibited a significant growth rate, reaching approximately 1000 publications at 2018. For publications with the title of “PEC,” the yearly number has exceeded 1300 at 2018. Obviously, the interest in PEC cells continues to maintain a marvelous pace as they remain widely employed in the advancement of solar fuel systems.
How to Design an Efficient Photoelectrodes
The development of suitable techniques to fabricate high-efficiency photoelectrode for PEC water splitting is crucial. To date, there are many methods, which have been developed to prepare photoelectrodes for use in solar water oxidation.
First, the morphologies of the materials used as a photoelectrode such as shape, size, and particle contact strongly affect their interfacial energetics, kinetics, and charge transport properties as well as reactive sites.
Second, nanocomposites provide a powerful route to overcome limitations in the current studies of single material systems for water splitting, where the photoelectrochemical performance of photoelectrode can be significantly improved by the choice of proper interactions of constituents.
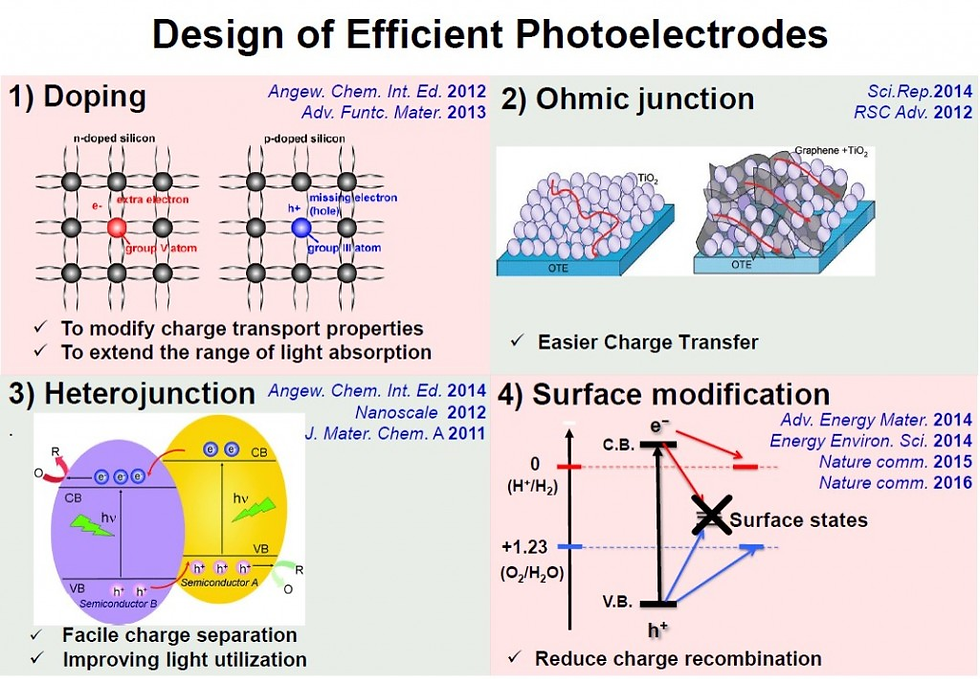
Third, doping with the metal (such as W, Cu, Zn, Ti, Mo, Ag, etc.) or nonmetal (such as P and N) materials can change the electrical and optical properties of the semiconductor materials used as a photoelectrode.
Finally, decoration of the surface of the photoelectrode with various oxygen evolution cocatalysts (OECs) such as Co–Pi, Co3O4, CoO, and NiFe improves the kinetic for oxygen evolution and provides unique active sites for catalytic reactions, thereby strongly enhancing the photocurrent density.


Comments