Our Research
- zohrehmasoumi17
- Apr 27, 2022
- 6 min read
Updated: May 3, 2022
Our research is aimed at providing materials chemistry solutions to address the problems of societal importance. Our current interests are mainly focused on the design of advanced materials for activating small molecule transformation reactions that are relevant to renewable energy conversions and commodity chemical productions. We design new materials tailored at a molecular level in a function-oriented manner, utilize spectroscopy, microscopy, and theoretical calculations to characterize materials at an atomic level, and explore these materials in a variety of catalytic reactions to establish the structure–property relationship.
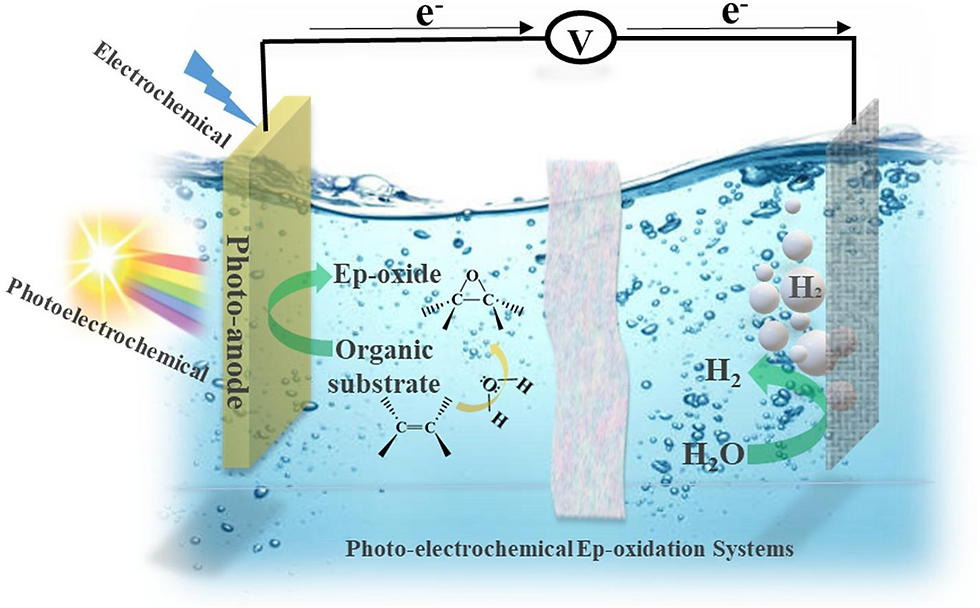
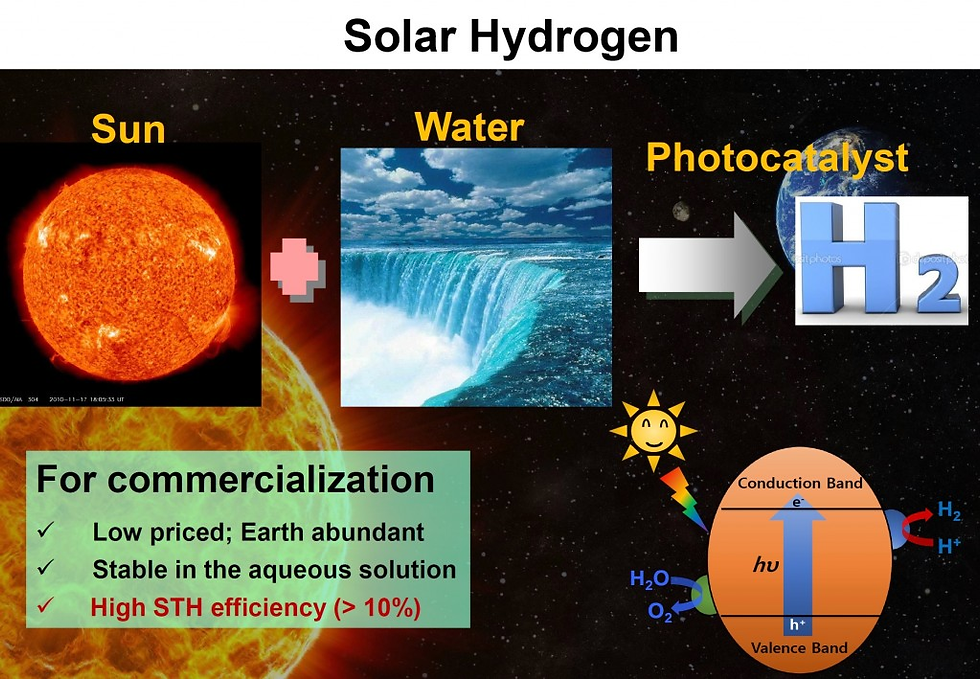
Our specific target reactions in renewable energy conversion area include oxygen reduction reaction and fuel oxidation reactions for fuel cells and oxygen evolution reaction and hydrogen evolution reaction for water splitting.
We use various strategies have been reported to improve the PEC performance of photoelectrodes such as nanostructures, doping (e.g., doped metals such as Ti, Mo, W, ... or non-metal doped such as S, P, ...), heterostructures (e.g., α-Fe2O3/WS2, WO3/BiVO4, and ZnO/Cds), and co-catalyst (e.g., apply Co-Pi or NiFe on the surface).

Some of our publication is...
The role of doping molybdenum (Mo) and back-front sides illumination in enhancing the charge separation of α-Fe2O3 nanorod photoanode toward solar water splitting
Abstract:
Hematite (α-Fe2O3) has abundant reserves and an appropriate bandgap of ∼2.1 eV for H2 production using water splitting. However, its potential application to solar water splitting is seriously limited by the extremely high charge recombination rate of the charge carriers. The present work investigated the photoelectrochemical (PEC) performance of molybdenum (Mo) doped α-Fe2O3 thin-film electrodes. The Mo doped α-Fe2O3samples were prepared by a hydrothermal method and then loaded the different ratio of molybdenum to optimize the photo current density. Morphological characterization and crystal structure identification were measured by FE-SEM, RAMAN, XRD, XPS and UV-vis analyses. After doping Mo on the α-Fe2O3, the photocurrent density significantly enhanced. The optimized Mo amount doped α-Fe2O3 film (10% Mo-doped α-Fe2O3) had a photocurrent density of 0.75 and 1.2 mA.cm-2 in the front and back side illumination at 0.6 V versus Ag/AgCl under 100 mWcm-2 and a 1 M (pH=12) aqueous NaOH solution as the electrolyte respectively, which are ~ 10 and 17 times higher than that of the pure α-Fe2O3 photoanode. The doping of Mo onto pure α-Fe2O3 caused a donor concentration increase (1.3-fold), a space charge layer reduction (1.2-fold), and a flat band potential decrease (1.6-fold), which improved the photoelectrochemical efficiency.

Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets
Abstract
Graphene-ZnO nanocomposites with different morphologies (nanoparticles and nanorods) were synthesized by alkali precipitation and solvothermal process. The influence of graphene nanosheets on photocatalytic and photoelectrochemical (PEC) activity of graphene-ZnO nanorods (ZnO/GNR) and graphene/ZnO nanoparticles (ZnO/GNP) was investigated. ZnO/GNR showed greatly increased photocatalytic degradation of methyl orange (MO): 2-, 2.5- and 3.5-fold higher under UV light and 1.6-, 4.7-, and 11-fold higher under visible light than that for ZnO/GNP, ZnO NR and ZnO NP, respectively. In addition, ZnO/GNR showed remarkable photocorrosion suppression (∼0.5%) compared to 10, 35 and 45% for ZnO/GNP, ZnO NR and ZnO NP, respectively. Furthermore, PEC results indicated a remarkable 6-fold enhancement photocurrent density for ZnO/GNR compared to ZnO NR. This enhancement is attributed to the crucial role of graphene in the electron transfer from ZnO NR into graphene sheets increasing the electron lifetime, leading to a minimized charge recombination.

Ultrasonication-assisted liquid-phase exfoliation enhances photoelectrochemical performance in α-Fe2O3/MoS2 photoanode
Abstract
This study successfully manufactured a p-n heterojunction hematite (α-Fe2O3) structure with molybdenum disulfide (MoS2) to address the electron–hole transfer problems of conventional hematite to enhance photoelectrochemical (PEC) performance. The two-dimensional MoS2 nanosheets were prepared through ultrasonication-assisted liquid-phase exfoliation, after which the concentration, number of layers, and thickness parameters of the MoS2 nanosheets were respectively estimated by UV–vis, HRTEM and AFM analysis to be 0.37 mg/ml, 10–12 layers and around 6 nm. The effect of heterojunction α-Fe2O3/MoS2 and the role of the ultrasonication process were investigated by the optimized concentration of MoS2 in the forms of bulk and nanosheet on the surface of the α-Fe2O3 electrode while measuring the PEC performance. The best photocurrent density of the α-Fe2O3/MoS2 photoanode was obtained at 1.52 and 0.86 mA.cm−2 with good stability at 0.6 V vs. Ag/AgCl under 100 mW/cm2 (AM 1.5) illumination from the back- and front-sides of α-Fe2O3/MoS2; these values are 13.82 and 7.85-times higher than those of pure α-Fe2O3, respectively. The results of electrochemical impedance spectroscopy (EIS) and Mott-Schottky analysis showed increased donor concentration (2.6-fold) and decreased flat band potential (by 20%). Moreover, the results of IPCE, ABPE, and OCP analyses also supported the enhanced PEC performance of α-Fe2O3/MoS2 through the formation of a p–n heterojunction, leading to a facile electron–hole transfer.
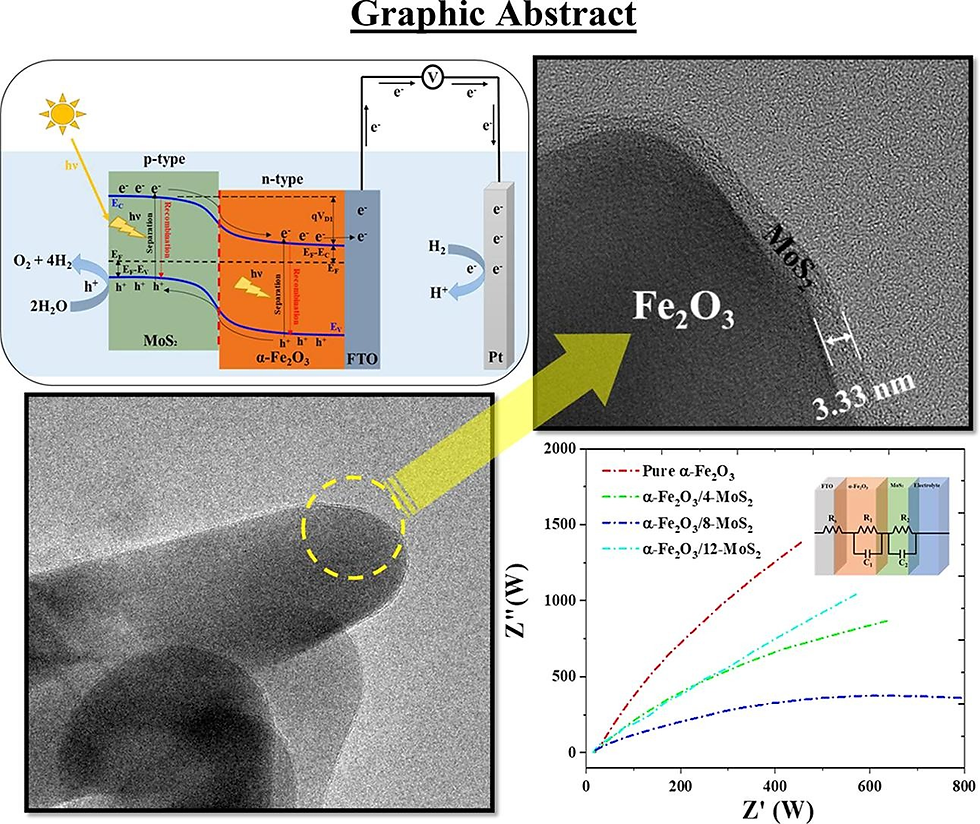
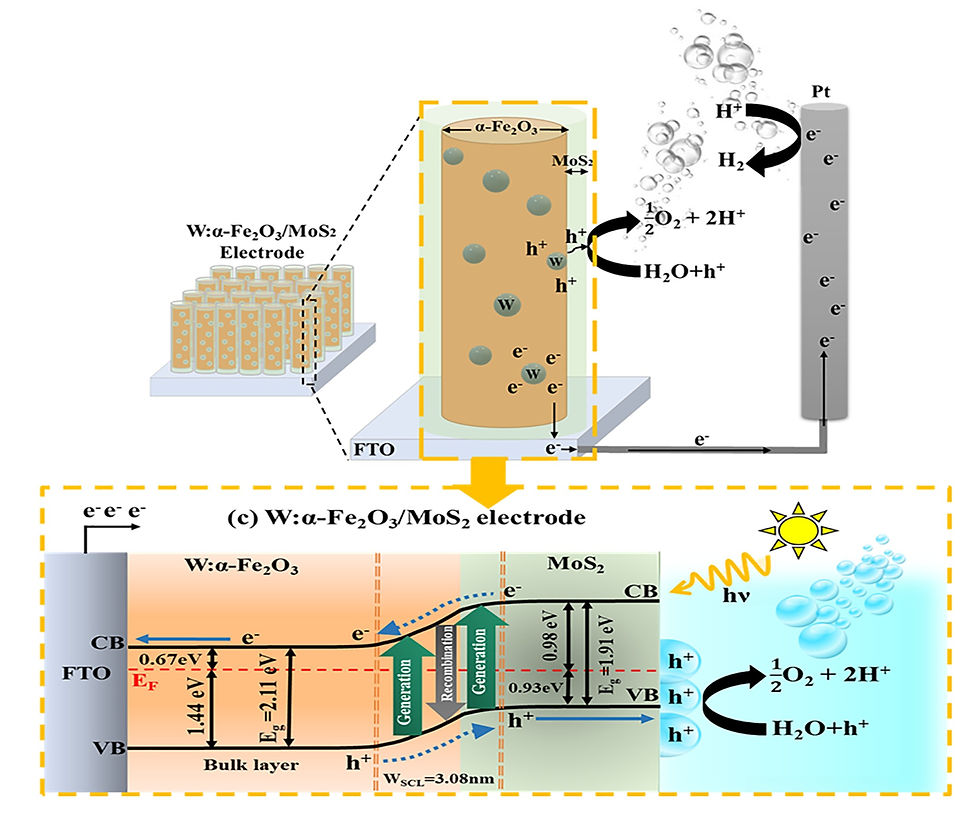
Simultaneous Enhancement of Charge Separation and Hole Transportation in a W:α-Fe2O3/MoS2 Photoanode: A Collaborative Approach of MoS2 as a Heterojunction and W as a Metal Dopant
Abstract
In this study, a facile approach has been successfully applied to synthesize a W-doped Fe2O3/MoS2 core–shell electrode with unique nanostructure modifications for photoelectrochemical performance. A two-dimensional (2D) structure of molybdenum disulfide (MoS2) and tungsten (W)-doped hematite (W:α-Fe2O3) overcomes the drawbacks of the α-Fe2O3 and MoS2 semiconductor through simple and facile processes to improve the photoelectrochemical (PEC) performance. The highest photocurrent density of the 0.5W:α-Fe2O3/MoS2 photoanode is 1.83 mA·cm–2 at 1.23 V vs reversible hydrogen electrode (RHE) under 100 mW·cm2 illumination, which is higher than those of 0.5W:α-Fe2O3 and pure α-Fe2O3 electrodes. The overall water splitting was evaluated by measuring the H2 and O2 evolution, which after 2 h of irradiation for 0.5W:α-Fe2O3/MoS2 was determined to be 49 and 23.8 μmol.cm–2, respectively. The optimized combination of the heterojunction and metal doping on pure α-Fe2O3 (0.5W:α-Fe2O3/MoS2 photoanode) showed an incident photon-to-electron conversion efficiency (IPCE) of 37% and an applied bias photon-to-current efficiency (ABPE) of 26%, which are around 5.2 and 13 times higher than those of 0.5W:α-Fe2O3, respectively. Moreover, the facile fabrication strategy can be easily extended to design other oxide/carbon-sulfide/oxide core–shell materials for extensive applications.
A review on metal-organic frameworks photoelectrochemistry: A headlight for future applications
Abstract
Photoelectrochemistry is an advancing interdisciplinary field, dealing with the chemistry and physics of photo-driven reactions at solid/liquid interfaces. This field requires collaboration between the (electro) chemists, physicists, spectroscopists, theorists, as well as materials and electronic scientists. MOFs, defined as a type of porous material with unique atomic ordering, display exception appearance superior efficiency for photoelectrochemical (PEC) applications. Recent applications of photoelectrochemistry at the metal-organic framework (MOFs)/electrolyte interfaces provide a sustainable and renewable strategy for light-harvesting that boosts ΔG < 0 reactions, without the genuine storage of chemical energy, while the radiant energy rate up a different leisurely reaction. However, so many fundamental and practical studies are yet to be carried out in this field. In this review, we have provided a comprehensive discussion of the fundamental concepts and photoelectrochemistry applications of the MOFs, in the following, challenges, potential, and opportunities are systematically summarized and discussed. Does this review provide answers to the following questions: How to prepare MOFs thin films as photoelectrodes? What are some important photophysical properties of MOFs-based photoelectrodes focused on the band structures, charge transfer type, kinetic of charge transfers, and light harvesting properties? How to modify the MOFs interface with other materials for enhanced PEC responses? How are MOFs utilized in PEC-based reactions? What are the experimental and theoretical techniques that allow us to obtain fundamental insights into photoelectrochemistry at MOFs? What are the design strategies for integrated MOF-based photoelectrodes? Which MOF-based PEC application has been further developed? We hope this review could assist researchers to deeper to understand the key features of MOFs for PEC applications and develop practical strategies for advancing the solar-powered chemical plants solar-powered chemical plants that will advance our society in the prolonged. We have tried to prepare a summary of what will be discussed in this review in the form of a map according to the Scheme 1 to better understand the concepts.

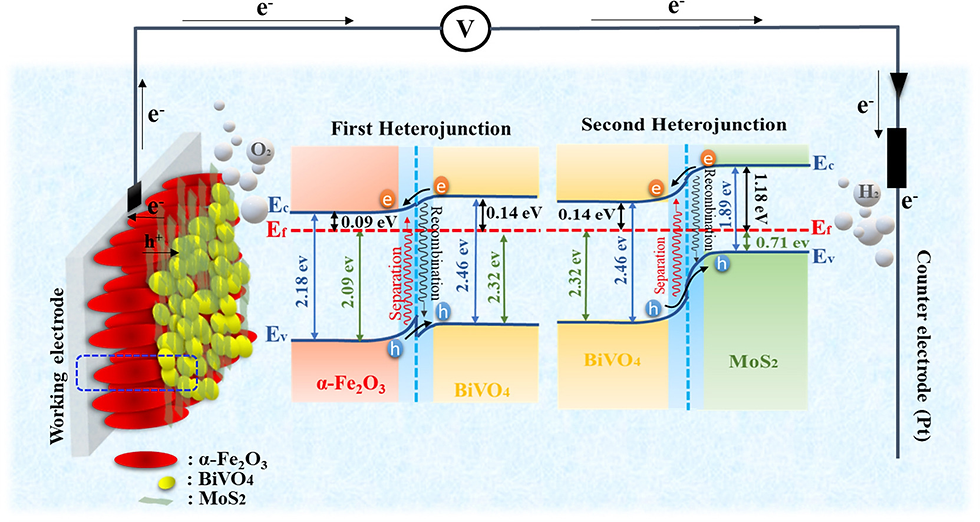
Unified surface modification by double heterojunction of MoS2 nanosheets and BiVO4 nanoparticles to enhance the photoelectrochemical water splitting of hematite photoanode
Abstract
Metal oxide semiconductors are among the most promising photoelectrode materials for solar water splitting, mainly due to their robustness in aqueous solutions. In this work, a multi heterojunction with metal oxides is fabricated to enhance the photoelectrochemical (PEC) water splitting through unified surface modification. The two-dimensional MoS2 nanosheets are synthesized through the liquid-phase exfoliation (LPE) process. The characteristics of the MoS2 nanosheets for improving light harvest and charge separation, including thickness, number of layers, and concentration, are all estimated using the proper techniques. The best photocurrent density of the α-Fe2O3/BiVO4/MoS2 at 1.23 V vs. RHE under 100 mW/cm2 (AM 1.5) illumination that was identified around 15 times greater than that of the α-Fe2O3 photoanode. The α-Fe2O3/BiVO4/MoS2 electrode shows the highest donor concentration value (4.36E+26 m3) with the lowest flat band potential (0.15 V) among all the prepared electrodes. Furthermore, the obtained hydrogen and oxygen production in 2 h irradiation for α-Fe2O3/BiVO4/MoS2 is 46.5 and 22.3 μmol cm−2, respectively.
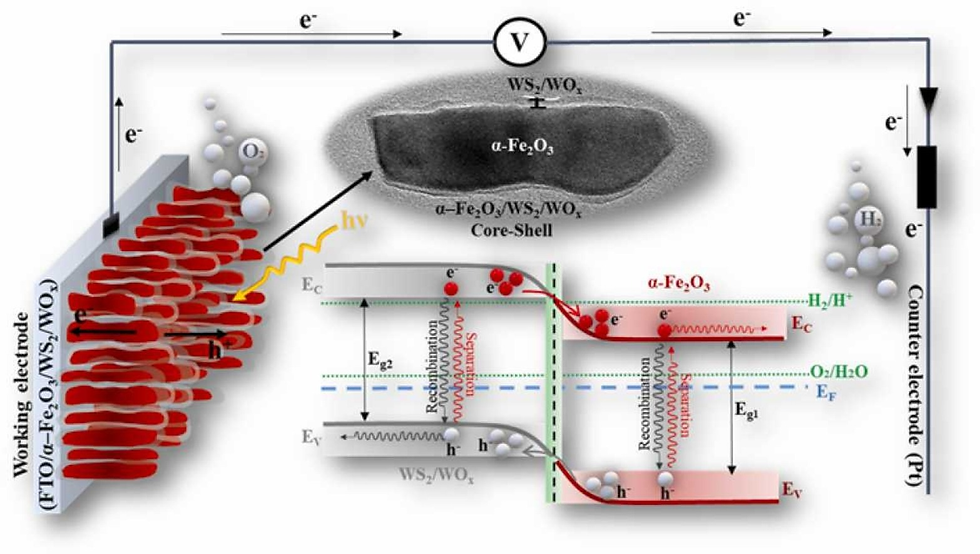
Efficient and stable core-shell α–Fe2O3/WS2/WOx photoanode for oxygen evolution reaction to enhance photoelectrochemical water splitting
Abstract
In the present work, the WS2 nanosheets were prepared through a liquid-phase exfoliation method (LPE). Various techniques were then used to characterize thickness, length, and concentration of these nanosheets. WS2 nanosheets were also loaded on α–Fe2O3 photoanodes to prepare core-shell structured α–Fe2O3/WS2/WOx photoanodes. These core-shell structured α–Fe2O3/WS2/WOx nanorods have advantages of effective separation, decreased recombination of photo-generated electron-hole pairs, and increased electron transport properties, resulting in improved PEC performance. The best photoanode (α–Fe2O3/#4-WS2/WOx) had photocurrent densities of 0.98 and 2.1 mA cm−2 (with the lowest onset potential 0.54 VRHE and 0.47 VRHE) under front and back-side illumination, respectively, at 1.23 VRHE under 100 mW cm−2, which were about 13 and 30-fold higher than those of pure α-Fe2O3 photoelectrode. Furthermore, H2 and O2 production of α–Fe2O3/#4-WS2/WOx photoanode were 32 μmol.cm−2 and 15.3 μmol.cm−2, respectively at 1.23 VRHE under 100 mW cm−2 after 2 h.

Comments